INTRODUCTION
Membranous nephropathy (MN) is a kidney disorder characterized by progressive thickening of the glomerular basement membrane, that causes nephrotic syndrome, leading to impaired chronic kidney function. Nephrotic syndrome can be caused by various underlying conditions that result in damage to the filtering units of the kidneys, known as nephrons, producing massive proteinuria, hypoalbuminemia, edema and hyperlipidemia. Between 30 to 40% of cases of nephrotic syndrome in adults are due to membranous nephropathy and is the leading cause of nephrotic syndrome in non-diabetic adults [1,2].
The exact cause of primary membranous nephropathy is often idiopathic (unknown) in about 70% of the cases. However, certain secondary causes and associations that have been identified include autoimmune diseases (systemic lupus erythematosus); autoimmune thyroid disease; infections like hepatitis B or C; medications like nonsteroidal anti-inflammatory drugs, penicillamine; malignancies as solid tumors and hematologic malignancies and others as described in Table 1.

In both cases, the presence of immunoglobin and complement- containing immune deposits of antigen-antibody complexes between the glomerular basement membrane and podocytes are responsible for the podocyte damage [5]. The exact triggers for the immune response leading to the formation of these complexes are not fully understood, but genetic factors may influence the susceptibility to such immune dysregulation.
Genetic factors
Individuals with a family history of kidney disease, including MN, may be at a higher risk of developing the condition, indicating a genetic predisposition.Several genetic factors have been implicated in the development of membranous nephropathy and mutations or variations in these genes may contribute to the abnormal immune response and the formation of immune complexes in the glomeruli, leading to inflammation and damage [6].
Associated genes
Genome-wide association study’s findings explain how autoantigens such as PLA2R trigger an autoimmune response [7]. Reports of a study group of 3 cohorts of total 556 European white descent patients with idiopathic membranous nephropathy diagnosis and 2,338 controls [8], identified HLA-DQA1 allele on chromosome 6 that could facilitate autoantibody development targeting not only PLA2R1 but also other antigens. The findings suggest that an interaction between genetic variants of immune-system proteins and glomerular components could be the trigger. HLA-DQA1 is HLA class II gene part of a heterodimer consisting of an alpha chain (DQA) and a beta chain (DQB), both anchored in the membrane and forming the antigen-presenting groove. Sequence variants in HLA-DQA1 chain contain polymorphisms that determine peptide binding specificities. If there is an alteration in the shape of the peptide groove, could therefore change its conformation, alter the immunogen presentation or amino acid sequence [9,10].
At this moment evidence suggests PLA2R1 is the most important target currently, and targeting it with autoantibodies is believed to be a major mechanism in MN development. There is some evidence suggesting a potential role for other genes in MN pathogenesis, like NFKB1, IRF4, that might be indirect or involved in specific pathways related to MN development. However, the evidence is not as strong as for PLA2R1. [15].
SNPs and MN
There was a significant association of MN with a single-nucleotide variation in the class II HLA-DQA1 locus rs2187668 and rs4664308 of PLA2R1, where patients with this risk alleles had an 11.13-fold higher risk of MN. Patients with homozygous rs9272729 of HLA-DQA1 and heterozygous PLA2R rs17830558 had an 80-fold higher risk of developing MN. HLA-DRB1 was also studied and risk alleles found were DRB1*1501 and DRB1*0301, also class II HLA genes, both with a higher risk of developing MN, ORs of 4.65 and 3.96 respectively [11,12].
Environmental factors
While genetic factors play a role, environmental triggers also contribute to the development of membranous nephropathy. Infections, medications (nonsteroidal anti-Inflammatory drugs and certain antibiotics), and exposure to certain toxins have been identified as potential environmental factors that can induce or exacerbate the condition, as has been reported in various studies in China, of MN cases in urban areas, with patients exposed to environmental pollution, have measurements of high levels of Th17 cytokines due to exposure to pollutant particles in the air (fine particle that are 2.5 micrometers or less in diameter known as Particulate matter 2.5 or PM2.5, produced by combustion, dust or smoke) can alter microRNA expression, thereby promoting the exposure of PMN autoantigens [13,14].
Antigens and antibodies
In MN the pathophysiology involves the formation of immune complexes within the glomerular basement membrane, that consist of antibodies and antigens. This antigen-antibody complexes activate complement system, generate C5b-C9 membrane attack complex, releasing cytokines, oxidants, proteases and disrupting podocyte structure and depositing between the glomerular basement membrane, increasing extracellular matrix, leading to inflammation and damage to the glomeruli [16,17]. Podocytes are differentiated cells with limited regenerative capacity and after damage, albumin permeability increases. Prolonged exposure to albumin provokes an inflammatory response and induces podocyte cell death by apoptosis [18].
Potential biomarkers
In 2009 PLA2R, M-type phospholipase A2 receptor and its antibody was discovered. PLA2R is a transmembrane glycoprotein expressed on the surface of podocytes, and is the primary disease antigen in primary MN [19, 20].
Since then and thanks to new technologies in kidney biopsy tissue, laser microdissection and mass spectrometry, a series of other target antigens have been discovered, for both primary and secondary MN. Some of these antigens have clinical diseases identified, as described in table 2.
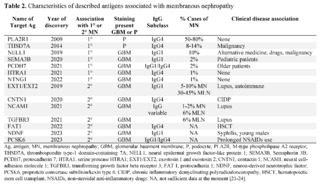
The concept of identifying an antigen in MN is that it accumulates or deposits in the glomerulus and this antigen is different and unique for each case. By means of immunohistochemistry or immunofluorescence, sub-epithelial deposits can be evidenced in the walls of the capillaries of the glomeruli. These antigens produce circulating antibodies, which can be measured in blood by Western blot techniques and thus confirmed. It is not yet easy to measure each of these antigens in the blood in all centers, but this opens a window of opportunities for identification in the clinic, and in the future, using renal biopsy for those cases that could not be identified through these tests [25].
For an antibody to be detected in the blood, it requires that there is a sufficient quantity in the circulation, which means that there would already be significant damage to the podocytes, which will allow the passage of albumin, with the consequent appearance of proteinuria. and presence of edema [26,27]. In some centers, testing is available to look for Anti-PLA2R antibodies in patient blood samples, using immunofluorescence and ELISA techniques [28].
It is important to note that in some cases of MN, neither anti-PLA2R or anti-THSD7A antibodies, or other antigens may be detectable, and the cause of the disease may be secondary to other underlying conditions, such as infections, autoimmune diseases, or medications. In these cases, antigens derived from infectious, autoimmune, environment or drugs, or circulating immune complex travel to the glomerulus and localizes in the subepithelial space. Also, tumor antigens can produce a humoral immune response that can consequently lead to secondary MN [29,30]. In the near future, some other techniques, as Cell-free DNA (cfDNA) testing, also known as liquid biopsy, a non-invasive diagnostic approach that involves analyzing fragments of DNA circulating freely in the bloodstream, could be helpful in identifying autoimmune diseases like membranous nephropathy, as it has also shown promise in identifying autoimmune diseases, like systemic lupus erythematosus [31].
Previously, the classification of MN established the difference between primary and secondary causes. In view of the discovery of these new antigens and associations with specific genes involved in the pathophysiology of MN, experts recommend that a new classification be promoted (Mayo Clinic consensus report on membranous nephropathy: proposal for a novel classification) [32], based on the specific antigens and whether there is an association with disease or use of medications established.
The discovery of these new antigens and their corresponding antibodies allows the development of diagnostic tests in serum that could at some point reduce the requirement for kidney biopsies and monitor the effectiveness of treatment or the evolution of the disease in terms of remission. While anti-PLA2R1 remains the most established target in MN diagnosis, exploring other antigens like NELL, THSD7A, and others holds promise for improving diagnostic accuracy.The understanding of the antigens involved in MN is an area of active research, and additional antigens may be identified as our knowledge of the disease continues to evolve.
